- REACH
- CLP
- COSMETICS
- ADR
- PMC
- BIOCIDES
- DETERGENT AND CLEANING PRODUCTS
- SAFE & SUSTAINABLE
- CAM
- ENVIRONMENTAL LABELLING
- ECOLABEL
- WASTE SORTING LABELLING
- SINGLE USE PLASTICS
- GLOBAL RECYCLE STANDARD
- REMADE IN ITALY
- COMPOSTABILE
- VENDITE ON LINE
- FOOD DIETARY SUPPLEMENTS
- EXPLOSIVES PRECURSORS
- PIC
- ReMaF
- FOOD CONTACT MATERIALS
- Training
- News
How to import cosmetics in EU
To import cosmetic products into Europe from an Extra EU country, it is necessary to comply with Regulation (EC) 1223/2009.
Here are some basic points to evaluate the supplier, the product and the correct strategy.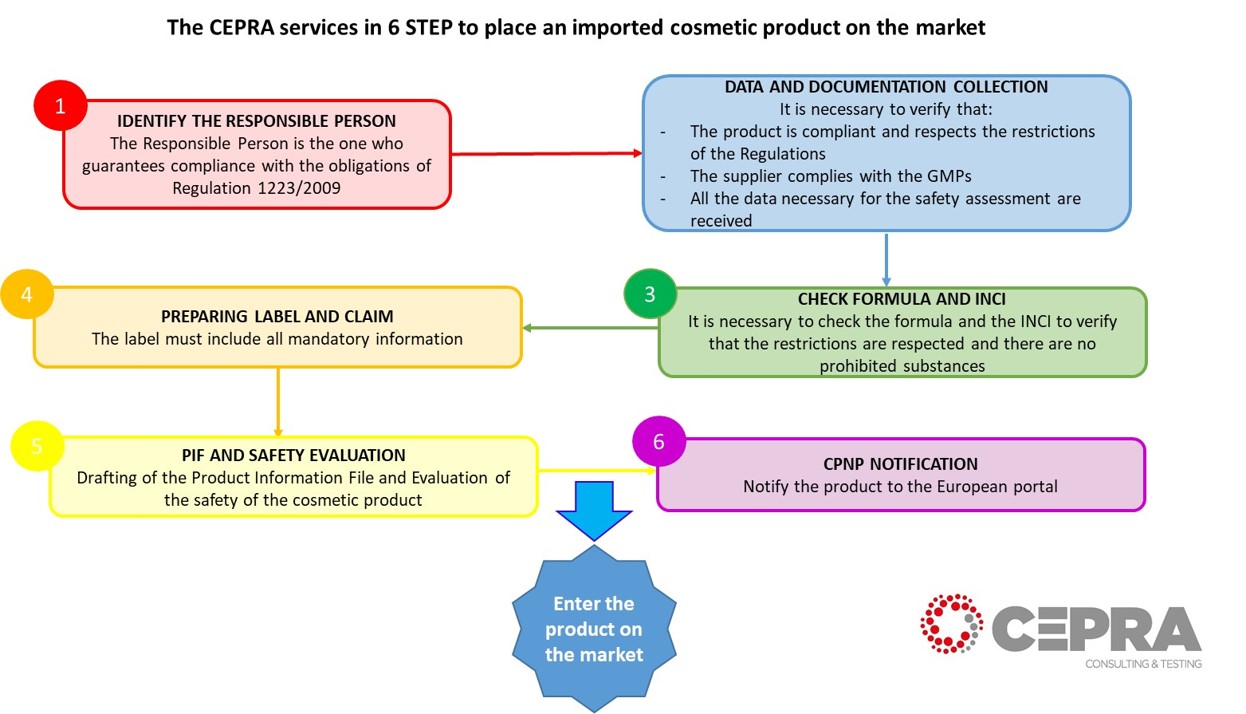
STEP 1: IDENTIFY THE RESPONSIBLE PERSON
The importer is the Person Responsible for the placing on the market of a cosmetic product in Europe, can identify a person within the European Union who assumes the role of Responsible Person.
STEP 2: DATA AND DOCUMENTATION COLLECTION
Ask your supplier for documentation to verify that the legal requirements required by Regulation 1223/2009 are respected. It is necessary to collect the documentation on the product.
The requested data are:
- Qualitative-quantitative formula
- INCI
- Batch analysis certificate (chemical, physical and microbiological analysis)
- Safety data sheets and data sheets of raw materials
- Stability test and challenge test (when necessary)
- Packaging Information (Compatibility Test)
- Any necessary analysis
STEP 3: CHECK FORMULA AND INCI
Once the formula has been obtained from the supplier, it is necessary to verify that it complies with Regulation 1223/2009 and its annexes. It is necessary to verify that there are no banned substances and that legal restrictions are respected.
STEP 4: PREPARING LABEL AND CLAIM
Verify that the label contains all the information required by the Art. 19 of the Regulations and that the claims used are appropriate.
STEP 5: PIF AND SAFETY EVALUATION
Before placing a cosmetic product on the market it is necessary to draw up the PIF (Product Information File), this document serves to collect all the information available on the cosmetic product, such as:
- Qualitative-quantitative formula
- Label
- GMP declaration of conformity and non-animal testing
- Manufacturing method
- Batch analysis certificate (chemical, physical and microbiological analysis)
- Safety data sheets and data sheets of raw materials
- Stability test and challenge test (when necessary) and any necessary analysis
- Packaging Information (Compatibility Test)
After evaluating all this information, it will be possible to draw up the safety assessment of the cosmetic product.
STEP 6: CPNP NOTIFICATION
The product must be notified on the CPNP (Cosmetic Product Notification Portal). This step allows you to place the product on the market, notifying the product to the authorities. The cosmetic regulation 1223/2009, according to article 13 defines the rules and obligations. Only one notification is required to access the EU market (31 countries) and the notification is made by the responsible person.
Once all these steps have been completed, you can enter the product in the European market.
CEPRA srl can support you in all the steps to put the cosmetic product on the market.