- REACH
- CLP
- COSMETICS
- ADR
- PMC
- BIOCIDES
- DETERGENT AND CLEANING PRODUCTS
- SAFE & SUSTAINABLE
- CAM
- ENVIRONMENTAL LABELLING
- ECOLABEL
- WASTE SORTING LABELLING
- SINGLE USE PLASTICS
- GLOBAL RECYCLE STANDARD
- REMADE IN ITALY
- COMPOSTABILE
- VENDITE ON LINE
- FOOD DIETARY SUPPLEMENTS
- EXPLOSIVES PRECURSORS
- PIC
- ReMaF
- FOOD CONTACT MATERIALS
- Training
- News
Labelling: info & instructions
INFORMATION REQUIRED ON THE LABEL
The label shall be in accordance with Article 19 of Regulation (EC) No. 1223/2009 of the European Parliament and of the Council.
The information must be given in the language of the country in which you put your cosmetics on the market.
Cosmetic products may be made available on the market only if the container and packaging of cosmetic products bear the following information in indelible, easily legible and visible:
- The name and the address of the company (Responsible Person).
- It is a legal requirement to state the net contents of a product on the pack; that is, the quantity of product at the time it is filled into the packaging. For cosmetics, it is shown in grams (g) or millilitres (ml) for solids or liquids respectively. A contents declaration is not required for products whose contents are below 5 g or 5 ml, for single use packs such as sachets or capsules, or for free samples. If products are sold as a collection of items, this should be stated; for example, 10 sachets. The "" mark must be shown if the product is filled according to the "average fill system" which is defined in weights & measures legislation.
- A “date of minimum durability” ("best used before the end of") or a “period after opening” to show for how long the product may be kept or used.
- Any warnings That might be Necessary on how to use the product safely.
- A reference (batch number) for product identification.
- Country of origin (for products imported into the EU).
- What the product is (if not obvious from its appearance).
- An ingredients list, in decreasing order of weight of the ingredients. This is mainly intended for people who have been diagnosed with an allergy so that they may avoid ingredients to which they are allergic. The same ingredient names are used across the European Union and most countries worldwide so people are easily able to identify them.
Period after opening, expiry date
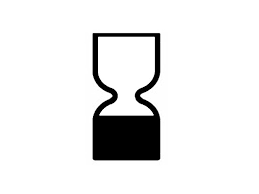
Any cosmetic product that has a shelf life lower than 30 months must show a expiry date (best before date). This can be shown using the "egg timer" symbol Followed by the dates.
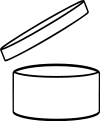
For products with as shelf life longer than 30 months, cosmetic products must show a "period after opening" time. That is, the time in days, months or years the product will remain in good condition after the consumer has used it for the first time. A symbol of an open cream jar is usually used instead of words and the time (usually) declared in months can be inside the symbol or alongside it.
INGREDIENTS
All ingredients used in a cosmetic, toiletry and perfumery product must be listed on the ingredients list. The same ingredient names are used in every European country and most countries worldwide.
An ingredients list should always appear in the same format and use the same conventions:
- It should be headed by the word INGREDIENTS.
- Ingredients should be listed in order of weight in the product
- Ingredient names are from the INCI naming system
- Perfume mixtures are labelled as "parfum" except for certain specific perfume ingredients which are listed by INCI name
- Colours use the Colour Index Number, or CI Number, an international naming system, for example "CI 15580"
- Ingredients are listed in descending order of weight . The ingredients in concentrations less than 1% may be listed in any order.
EXEMPTIONS
Where it is impossible from a practical standpoint label the information above, the following applies:
- The information is shown on a sheet, on a label, tape or card which is enclosed or attached to the cosmetic product;
- In the case of soap, bath balls and other small products, it is virtually impossible to give the information mentioned above, on a label, tape or card or in an enclosed leaflet, this information must appear on a notice in immediate proximity to the container in which the cosmetic product is exposed for sale.
- For cosmetics are not pre-packaged for cosmetics by the seller on the buyer's request or pre-packaged for immediate sale, Member States shall lay down the manner in which the information should be indicated.
- The language in which the information should be indicated is determined by the Member State in which the product is made available to the end user.
CEPRA s.r.l. provides solutions to label cosmetics in accordance with the Regulation n ° 1223/2009, to notify the products on the CPNP portal, create the PIF (Product Information File) and CPSR (Cosmetic Product Safety Report).